New FDA regulations for "gluten-free"
by Nick Rose, PCC Nutrition Educator
This article was originally published in August 2014
More than a quarter of Americans report they are cutting down on or eliminating gluten from their diet. The result? The market for gluten-free foods has skyrocketed over the past five years. Restaurants have developed gluten-free menus, big food companies have jumped onto the gluten-free bandwagon, and even beers and body care products are being labeled gluten-free.
As of this month, gluten-free claims must conform with new regulations by the U.S. Food and Drug Administration (FDA), requiring that foods labeled “gluten-free” must be free of wheat, barley or rye, and that “any unavoidable presence of gluten” must result in less than 20 parts per million (ppm) of gluten.
Why 20 ppm?
Many gluten-intolerant shoppers are surprised to learn that gluten-free foods are allowed to contain trace amounts of gluten. A zero ppm threshold, which sounds great in theory, would not be practical for food manufacturers that handle ingredients often processed in a facility also handling wheat. Also, it’s impossible to detect gluten at zero ppm.
The FDA’s gluten-free definition was constructed for the 1 percent of the U.S. population with celiac disease, the most severe form of gluten intolerance. Limited research suggests that celiacs safely can tolerate up to 10 mg each day of gluten (about 1/8 teaspoon) without measurable problems. Consuming 12 servings each day of foods containing the maximum 20 ppm (0.002 percent) still would be safe and below the 10 mg per day threshold.
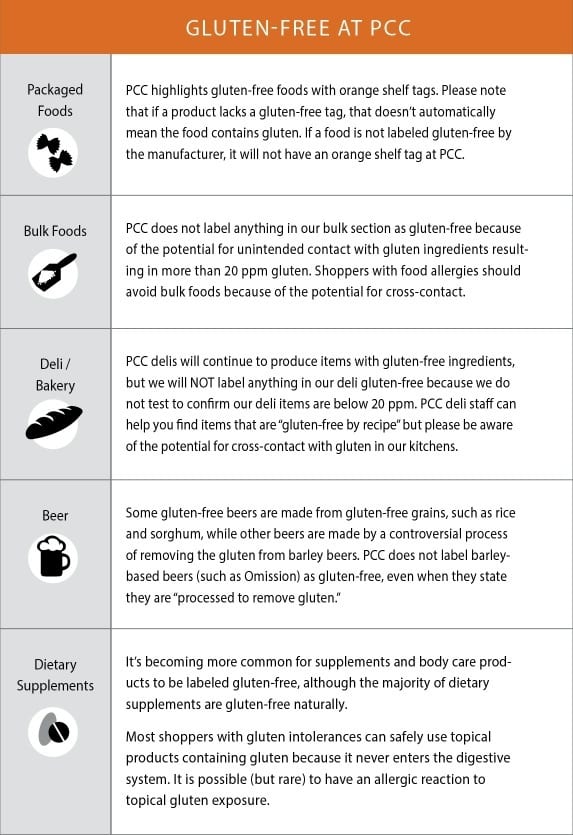
Impact of FDA rule
“The vast majority of companies already are following the guidelines for labeling gluten-free,” says Cynthia Kupper, a registered dietitian and executive director of the Gluten Intolerance Group. “Others are transitioning their labels now.” She predicts gluten-free label claims will continue to increase, as will gluten-free certification. Gluten-free certification by a third party will continue to give shoppers the greatest confidence in gluten-free label claims because most certifiers test below FDA’s 20 ppm requirement.
Meat and poultry are exempt from these guidelines, because they’re regulated by the U.S. Department of Agriculture, not FDA. Alcohol also is exempt. Meat, eggs and dairy always are gluten-free, even if the animals are fed gluten-containing grains. But gluten-free labels still are useful for packaged meats, which can contain gluten from seasoning mixes or other added ingredients. Dairy products are gluten-free naturally, but potentially could contain gluten from added ingredients, such as malted barley syrup, oats or modified food starch.
Please note that gluten-free claims are not health claims. They do not mean a food is any healthier for you. I don’t agree with the 100-percent gluten-free worldview that is endorsed by some nutritionists. Read more about common myths involving wheat and gluten in the article, “What’s Wrong with Wheat?”.
